Sarah Chapman looks at the latest Cochrane evidenceCochrane Reviews are systematic reviews. In systematic reviews we search for and summarize studies that answer a specific research question (e.g. is paracetamol effective and safe for treating back pain?). The studies are identified, assessed, and summarized by using a systematic and predefined approach. They inform recommendations for healthcare and research. on the safetyRefers to serious adverse effects, such as those that threaten life, require or prolong hospitalization, result in permanent disability, or cause birth defects. and effectivenessThe ability of an intervention (for example a drug, surgery, or exercise) to produce a desired effect, such as reduce symptoms. of the shingles vaccination for older adults.
Page last updated 04 October 2023.
Herpes zoster or ‘shingles’ is a painful condition caused by the reactivation of varicella zoster virus (VZV), the virus that causes chicken pox. Older adults are particularly susceptible to shingles, which can last for weeks or months and can significantly impact quality of life.
NHS shingles vaccination programme
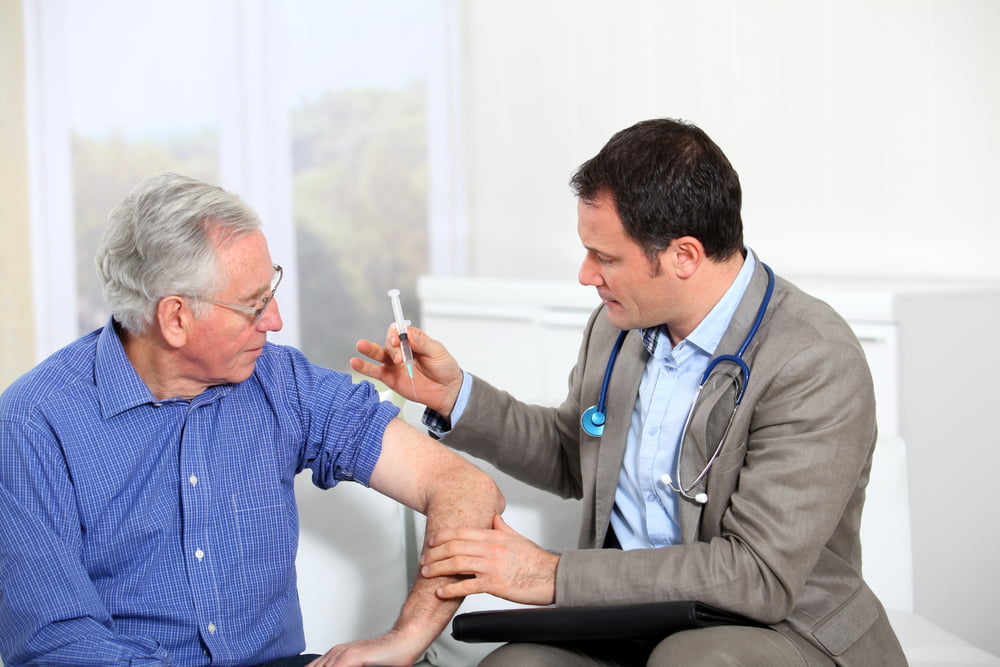
In the UK, the NHS recommends the vaccine for people at higher risk from shingles, including all adults turning 65, those aged 70 to 79 and those aged 50 and over with a severely weakened immune system. The NHS uses the attenuated live zoster vaccine (LZV). It is given as a single injection into the arm and, unlike the flu jab, it is given once only and at any time of year. There is also a newer vaccine against shingles, the recombinant zoster vaccine (RZV), which is given in two doses, two to six months apart. Both LZV and RZV have been approved for clinical use.
The latest evidence on shingles vaccination
A Cochrane ReviewCochrane Reviews are systematic reviews. In systematic reviews we search for and summarize studies that answer a specific research question (e.g. is paracetamol effective and safe for treating back pain?). The studies are identified, assessed, and summarized by using a systematic and predefined approach. They inform recommendations for healthcare and research. looking at the effectiveness and safety of vaccination to prevent herpes zoster in older adults was updated in October 2023. The review now includes 26 studies with 90,259 people, most aged sixty and over. Sixteen studies tested the LZV (single dose) and included 55,975 people; 10 studies tested the RZV (two doses with one two‐month interval between them), and included 34,284 people. The studies only included people who had no illnesses which compromise the immune system.
What does the research show?
For both LZV and RZV, there is moderate-certaintyThe certainty (or quality) of evidence is the extent to which we can be confident that what the research tells us about a particular treatment effect is likely to be accurate. Concerns about factors such as bias can reduce the certainty of the evidence. Evidence may be of high certainty; moderate certainty; low certainty or very-low certainty. Cochrane has adopted the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) for assessing certainty (or quality) of evidence. Find out more here: https://training.cochrane.org/grade-approach evidence that:
- LVZ (one dose) is probably effective – as older adults vaccinated with LZV had a lower rateThe speed or frequency of occurrence of an event, usually expressed with respect to time. For instance, a mortality rate might be the number of deaths per year, per 100,000 people. of shingles compared to those who received a placeboAn intervention that appears to be the same as that which is being assessed but does not have the active component. For example, a placebo could be a tablet made of sugar, compared with a tablet containing a medicine. vaccine. It would be necessary to vaccinate 50 healthy older adults with LVZ to prevent one episode of shingles.
- Vaccination with RZV (two doses) is also probably effective and is perhaps more effective than vaccination with LZV, since results show that 33 healthy older adults would need to be vaccinated to prevent one episode of shingles.
- There are probably more adverse effects (such as soreness at the injection site or headache) associated with vaccination with LZV and RZV than with placebo, but these are mostly mild to moderate and short-lived, lasting for one to three days.
- People vaccinated with LZV or RZV are probably no more or less likely to experience serious adverse events than those who have a placebo vaccination.
- In studies that tested RZV, which requires a second dose two months later, a small number of people (1 in 100) dropped out of the studies before getting the second dose of the vaccine – and it’s possible that these people dropped out due to side effects of the first dose.
There is more information about shingles and the shingles vaccination (including how to get the vaccine) on this NHS website.
de Oliveira Gomes J, Gagliardi AMZ, Andriolo BNG, Torloni MR, Andriolo RB, Puga ME, Canteiro Cruz E. Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic ReviewsIn systematic reviews we search for and summarize studies that answer a specific research question (e.g. is paracetamol effective and safe for treating back pain?). The studies are identified, assessed, and summarized by using a systematic and predefined approach. They inform recommendations for healthcare and research. 2023, Issue 10. Art. No.: CD008858. DOI: 10.1002/14651858.CD008858.pub5. Accessed 04 October 2023.
Sarah Chapman has nothing to disclose.


I had shingles about 3 years ago. It went over my left eye and also on the whole of the top half of my body. It also crossed the “centre line” which I believe it does not usually do. I did not seek treatment immediately but when I did, I was fortunate to stop any more damage to the eye area. I also received the shingles vaccination when it was safe to do so. Strangely enough, I do not remember having chicken pox as a child. I still suffer from unbelievable itching over my forehead and scalp. I take Gabapentin which eases the symptoms but does not stop it. Nothing the doctor has tried helps. Two years later I developed Balls Palsy on the opposite side which also had some lasting facial paralysis.
Hi, I have removed your reference to a website selling herbal treatment as we don’t allow commercial links.
Best wishes,
Sarah Chapman [Editor]