In this first evidence blog in our new series Evidence for Everyday Nursing, I’ve looked at a Cochrane reviewCochrane Reviews are systematic reviews. In systematic reviews we search for and summarize studies that answer a specific research question (e.g. is paracetamol effective and safe for treating back pain?). The studies are identified, assessed, and summarized by using a systematic and predefined approach. They inform recommendations for healthcare and research. which found no evidence to support routinely replacing peripheral venous catheters. This was then discussed in a #WeNurses tweetchat, summarised in the blog Getting evidence into nursing practice: replacing the routine.
Editors’ note 11 August 2022: these two related blogs from 2015 have been kept as an example of how evidence can lead to changes in practice, but also that practice change and adherence to guidelines doesn’t happen seamlessly or all at once. The Cochrane Review was updated again in January 2019 with the addition of two studies and no change to conclusions.
Routine can be a dangerous thing. It might be a very good thing to do something routinely, of course, but practices that are so entrenched that no-one questions them might not be the best thing at all.
What do you do when it comes to managing peripheral venous catheters (PVCs)? Change them routinely or only when there are clinical signs that this is necessary, such as blockage, pain, redness, infiltration, swelling, leaking or phlebitis?
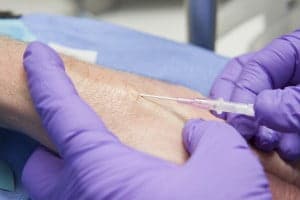
The UK’s epic3 National Evidence Based Guidelines recommend that short peripheral catheters should be replaced when clinically indicated, unless the patient is receiving parenteral nutrition peripherally. These guidelines cite the previous version of a Cochrane review, which has recently been updated. There were no new studies to add in 2015.*
The Cochrane Review Clinically‐indicated replacement versus routine replacement of peripheral venous catheters (updated January 2019) brings together the best available evidence from randomizedRandomization is the process of randomly dividing into groups the people taking part in a trial. One group (the intervention group) will be given the intervention being tested (for example a drug, surgery, or exercise) and compared with a group which does not receive the intervention (the control group). controlled trialsA trial in which a group (the ‘intervention group’) is given a intervention being tested (for example a drug, surgery, or exercise) is compared with a group which does not receive the intervention (the ‘control group’). (RCTs) comparing routine replacement of PVCs with re-siting them only when clinically indicated. The reviewers were interested in four main outcomesOutcomes are measures of health (for example quality of life, pain, blood sugar levels) that can be used to assess the effectiveness and safety of a treatment or other intervention (for example a drug, surgery, or exercise). In research, the outcomes considered most important are ‘primary outcomes’ and those considered less important are ‘secondary outcomes’.: catheter-related bloodstream infection; phlebitis; all-cause bloodstream infection; and cost. In 2015 they were able to include seven RCTs with 4895 patients in both acuteA health condition (or episodes of a health condition) that comes on quickly and is short-lived. and community settings (for the 2019 update, nine studies with 7412 people). Routine replacement was done at between 72 and 96 hours in five trialsClinical trials are research studies involving people who use healthcare services. They often compare a new or different treatment with the best treatment currently available. This is to test whether the new or different treatment is safe, effective and any better than what is currently used. No matter how promising a new treatment may appear during tests in a laboratory, it must go through clinical trials before its benefits and risks can really be known. and every 48 hours in two.
Here’s what they found:
- No difference in catheter-related bloodstream infection (which was between 0.0% and 0.3%)
- No difference in phlebitis rates (and this was the same whether infusions were continuous or intermittent)
- No difference between groups when analysed by the number of device days
- No difference in all-cause bloodstream infection (assessed in one trial)
- Lower cannulation costs in the clinically-indicated replacement group
How reliable is the evidence?
For the outcomes all cause bloodstream infection and phlebitis the evidence was assessed as moderate quality and for catheter-related bloodstream infection low quality (this has been changed to reflect the latest version of the review, in January 2019).
How much money could this save the NHS?
It has been calculated that if the clinically-indicated strategy was fully implemented in all NHS hospitals in England, the cost savings would be around £40 million over five years (Tuffaha et al, 2014;NICE, 2016)
What about catheter blockage?
Catheter failure due to blockage was more frequent in the clinically-indicated group, as would be expected – all of them fail eventually, but many last the length of treatmentSomething done with the aim of improving health or relieving suffering. For example, medicines, surgery, psychological and physical therapies, diet and exercise changes.. The reviewers point out that this outcome isn’t clinically meaningful; it just indicates that they were in place for longer in the clinically-indicated group. As the ‘treatment’ for a blocked catheter is to replace it, taking it out earlier wouldn’t reduce the need for replacement!
What was missing?
The review team planned to compare ‘the number of catheter re-sites per patient’, ‘pain’ and ‘satisfaction’, but these were not reported in the included studies (one small studyAn investigation of a healthcare problem. There are different types of studies used to answer research questions, for example randomised controlled trials or observational studies. added in 2019 reported on pain and found there may be no difference between groups). Five of the studies were conducted in Australia, one in the UK and one in India (the studies added in the 2019 update of the review were conducted in Brazil and China). The reviewers, though noting that the patients were ‘representative of those usually managed in healthcare,’ said it would be useful to see similar studies from other healthcare systems.
What does this mean for your practice?
The review found no evidence to support routinely changing PVCs. I guest-hosted a #WeNurses tweetchat on 17 November to discuss the evidence and practice. Find out more on the €WeNurses chat archive here and in my blog about the chat here.
Images may not be reproduced as they have been purchased from stock.com for Evidently Cochrane
References may be found here


