In this blog for researchers and people interested in trials, Catherine Houghton, senior lecturer at the National University of Ireland Galway, looks at the latest Cochrane evidence in trial methodology and explores the factors that impact on recruitment to randomised trials.
Page last checked 28 June 2023
Take-home points
What factors influence a person’s decision to take part in a trial? In short, the factors are many and not as straightforward as one would think. Randomised trials are needed for understanding the effects (both benefits and harms) of different healthcare interventions (such as medicines, types of surgeries, health promotion activities, etc.). Getting people to take part in trials can be difficult and if not enough people participate, then the trial will not provide the evidence that it set out to. Two previous systematic reviews examined strategies to improve recruitment (Treweek 2018, Gardner 2020). Treweek (2018) found that having an open trial (telling people what they receive in the trial) and phoning people who do not respond to a postal invitation, may be effective ways to improve recruitment. There was much less certainty that other strategies (such as developing participant information leaflets with feedback from users or providing information using videos) increase recruitment. By learning more about what influences a person’s decision to take part in a trial, we can provide advice on how best to include people in trials.
What did we do?
Our Cochrane Review “Factors that impact on recruitment to randomised trials in health care: a qualitative evidence synthesis” was published in October 2020. We wanted to find out what factors influence a person’s decision whether to take part in a trial. To do this we used qualitative evidence synthesis to bring together the findings from qualitative studies, and qualitative components of mixed methods studies, to explore the views and experiences of individuals invited to take part in a trial.
We included 29 studies in our review which captured the experience of 847 adults invited into a trial. We included people who had agreed to take part and well as those who declined. The studies originated in a number of countries including UK, Austria, Denmark, Germany, Sweden, The Netherlands, USA, Australia, Canada, New Zealand and Tanzania. We included people who had been invited to a number of different types of healthcare trials that we categorised as oncology (cancer), pregnancy and childbirth, medicine and surgery, mental health and health promotion.
People were invited to take part in the trial in a number of ways, but mainly by face-to-face invitation to participate during consultation with healthcare professionals, face-to-face invitation by research staff outside of a consultation, letter of invitation after being deemed eligible by healthcare professionals, posters and flyers and telephone recruitment.
What did we find out?
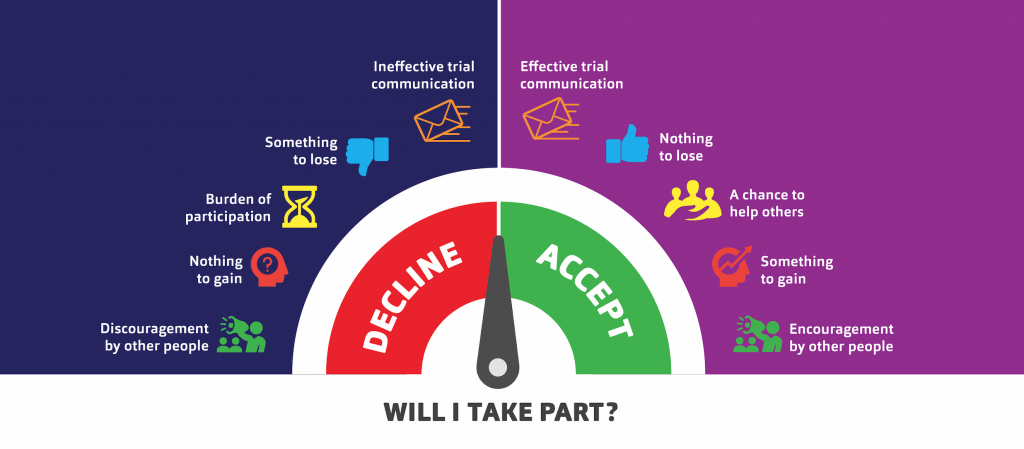
We identified a number of key factors that influence a person’s decision to take part in a trial. We broadly categorised these as trial influences, personal influences and the potential outcome of taking part in the trial.
Trial influences
In terms of trial influences, the way information about the trial was communicated could influence a person’s decision. Communicating face-to-face could be less confusing than written information, although written information was useful to refer to later. The person delivering the information should be approachable, trustworthy and knowledgeable, regardless of whether they are clinicians or researchers. The information should be robust but concise and free of jargon and include all key information about what participation will involve for that individual. It is important to consider when this information is communicated, so that the person being invited does not get confused between elements of the trial and their usual treatment and diagnosis.
Other trial influences relate to burden, financial incentives and what randomisation means for the person. If people needed to travel or participating in the trial required a big time commitment, people were less likely to participate. While financial incentives were welcomed, they were not seen as an important deciding factor. Understanding randomisation and the freedom to withdraw from the study was critical as those who did not fully understand these concepts, or who had a strong treatment preference, were less likely to agree to take part.
Personal influences
Personal influences include the influences of significant others, including family, friends, healthcare professionals and the media. Healthcare professionals in particular may influence decision-making as individuals placed huge trust in them. There was great potential for influence by healthcare professionals to impact on decision making.
Another personal influence relates to how at risk the person felt, with people commonly referring to feeling like a guinea pig. The level of risk was less about the trial itself, but what risk means to that person. By way of example, a person who feels in good health may feel they have “nothing to lose” by participating if they feel the trial was safe. Equally, a “healthy” person may not want to risk their health by taking part. For the person who has significant health problems, they may not wish to take the risk of taking part in a trial, or, they may feel desperate and again, feel that they have “nothing to lose” by taking part. This emphasises that perception of risk is a personal factor.
Potential outcome of taking part
The third category relates to the potential outcome of taking part in the trial. Understandably, people were more likely to take part if they could envisage a personal benefit such as access to new treatments, quicker access to services, better follow-up care and increased contact time with physicians. This was even more important for those who had been managing symptoms for some time and were desperate for relief. As well as personal benefits, people wanted to make a difference. We found this to be an important factor for those making the decision to participate in a trial. They had a desire to help others, wanted to “give back” to the community and had a genuine interest in contributing to science.
We have summarised the key factors as an infographic to highlight what influences the person deciding whether they should take part:
We assessed the confidence in our findings using the GRADE-CERQual approach (Lewin 2018) which showed moderate and high confidence in all of our findings except one, which related to influence of the media and internet – so we are less certain about these influences. This means our findings are relevant to many different types of trials. However, our findings are limited to mainly high-income countries and we only included adults with capacity to consent in our review.
What next?
While we assert that there is a lot of primary research in this area, there needs to be more research in lower-income countries, particularly in Africa and Asia. In addition, identifying factors that affect black, Asian and minority ethnic involvement in trials held in middle- and higher-income countries, as well as that of other under-represented groups, such as the socially disadvantaged, is needed. Also, we need further synthesis of the experiences of recruitment in the context of trials with children, and adults with impaired capacity to consent.
What the trial community needs most now is the development and testing of robust recruitment strategies that are individualised and participant-centred and draw directly from the experiences of those reported in this review. It is also important to consider the impact of recruitment on trial retention (Daykin 2018). You can read more in this blog by Katie Gillies and Derek Stewart: Retention to clinical trials: how can we keep participants involved?
We developed a set of questions that trialists can ask to guide their recruitment strategy. These questions encourage an approach to recruitment that considers characteristics of the trial, but also the characteristics of the people being invited; in a person-centred and individualised way. Ultimately the aim is to answer each person’s question of “what’s in it for me?”
You can listen to Catherine Houghton discuss these findings in a Cochrane podcast: What factors influence a person’s decision whether or not to take part in a randomised trial? Catherine is also an author on a Cochrane Review on Factors that impact recruitment to vaccine trials in a pandemic or epidemic (September 2023).
Join in the conversation on Twitter with @CochraneUK and @houghtoncath or leave a comment on the blog. Please note, we cannot give medical advice and we will not publish comments that link to commercial sites or appear to endorse commercial products. We welcome diverse views and encourage discussion. However we ask that comments are respectful and reserve the right to not publish comments we consider offensive.
Catherine Houghton has nothing to disclose.