Sarah Chapman and Selena Ryan-Vig highlight Cochrane evidence on COVID-19 and other health effects of the pandemic, with links to reviews, blogs and other Cochrane resources.
This blog was last updated on 25 May 2023.
While we’ve all been adapting to huge and sudden changes in our lives and healthcare workers have been meeting unprecedented challenges, scientists have scrambled to produce research evidence relevant to the pandemic. Cochrane is responding by producing rapid reviews of this new evidence on priority topics, and these are updated as new evidence emerges. They show that much of the research that has been done so far leaves us with more unanswered questions than answers, but we must hope that this changes as new studies are available to add to the reviews.
Here’s a round-up of some of the Cochrane evidence so far.
We have two separate blogs about the evidence on treating people with COVID-19:
- Treatments for moderate to severe COVID-19: Cochrane evidence
- Treatments for mild COVID-19: Cochrane evidence
On this page, there are sections on:
- Detecting COVID-19
- Measures to control the spread of COVID-19
- Things that increase people’s risk of suffering from severe COVID-19 illness
- Preventing COVID-19
- The impact of the pandemic on other areas of health and wellbeing
- Preventing and treating persistent symptoms after COVID-19 infection
- Vaccination
- Coronavirus (COVID-19): Special Collections
- Coming up…
.
Detecting COVID-19
Signs and symptoms of COVID-19
The Cochrane Review Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19 was updated for the second time in May 2022, with new evidence and changed conclusions.
You can read the blog: “Signs and symptoms of COVID-19: Cochrane evidence“.
Antibody tests for COVID-19
The Cochrane Review ‘Antibody tests for identification of current and past infection with SARS-CoV-2‘ was updated in November 2022, this time only looking at studies of commercially produced tests and how well the tests work over time. In people with COVID-19, antibody tests done one week after symptoms started found only 27% to 40% of infections. This improved after two weeks and was a lot better after three, when they picked up 93% of people with infections. The tests wrongly said people had COVID-19 (in those who didn’t) in just 1% of people. The authors note the results may be more relevant to people with more severe illness, as many people taking part in the research were in hospital with COVID-19.
The review authors say the evidence shows that antibody tests could be useful to detecting if someone has had COVID‐19, but the timing of the test matters. Some antibody tests may help to confirm COVID‐19 infection in people who have had symptoms for more than two weeks but who have been unable to confirm their infection by other means. Antibody tests may also be useful to find how many people have had COVID-19 in the past.
It’s uncertain how well the tests work for people with mild disease, or no symptoms, or for detecting antibodies due to vaccination,
Rapid point-of-care tests for diagnosing COVID-19 infection
Tests for diagnosing COVID-19 infection are important tools for helping reduce the spread of infection in communities, schools and workplaces, and have received a huge amount of attention in the press and on social media during the pandemic. The Cochrane Review Rapid, point‐of‐care antigen and molecular‐based tests for diagnosis of SARS-CoV-2 infection, has been updated for the second time in July 2022. The review looks at two types of test, antigen tests (including lateral flow tests or LFTs) and molecular tests. Both types of test use swab samples taken from the nose or throat, can be used outside of a specialist laboratory and provide results in less than two hours.
Lead author, Jac Dinnes, updated her blog COVID tests: how accurate are LFTs? when the review was updated in July 2022.
Key messages from the latest update:
- “Lateral flow tests (LFTs, also called rapid antigen tests) are most accurate when they are used in people who have signs or symptoms of COVID-19, especially during the first week of illness. People who test negative may still be infected.
- LFTs are considerably less accurate when they are used in people with no signs or symptoms of infection but do perform better in people who have been in contact with someone who has confirmed COVID-19.
- The accuracy of LFTs varies between tests that are produced by different manufacturers and there is a lack of evidence for many commercially available tests.”
Routine laboratory tests – how good are they for detecting COVID-19?
Routine blood tests, processed in laboratories, include counts of different types of white blood cells that help fight infection and identifying proteins (‘markers’) that can indicate general inflammation and organ damage. These are widely available and in some places might be the only tests available for diagnosing COVID-19.
A Cochrane Review Routine laboratory testing to determine if a patient has COVID‐19 (November 2020) has looked for evidence on the accuracy of these tests in people with suspected COVID-19 for diagnosing the disease and for prioritizing people for different levels of treatment. The review includes 21 studies looking at 67 routine laboratory tests for COVID-19, all in people who were patients in hospitals.
The bottom line: “Although these tests give an indication about the general health status of patients and some tests may be specific indicators for inflammatory processes, none of the tests we investigated are useful for accurately ruling in or ruling out COVID‐19 on their own.”
Screening for COVID-19
Screening people who have mild or no symptoms but have COVID-19, to find out if someone’s infected, is another strategy that has potential to help reduce the spread of infection, as those who are found to have the virus could then isolate, for example. A Cochrane rapid review on universal screening for SARS‐CoV‐2 infection was published in September 2020. The evidence base is currently very limited and highlights uncertainty about the effectiveness of screening for COVID-19. The review finds that:
“One‐time screening in apparently healthy people is likely to miss people who are infected. We are unsure whether combined screenings, repeated symptom assessment, or rapid laboratory tests are useful.
As more people become infected, screening will identify more cases. However, because screening can miss people who are infected, public health measures such as face coverings, physical distancing, and quarantine for those who are apparently healthy, continue to be very important.”
Here is a video summary.
Thoracic (chest) imaging tests for COVID-19
A Cochrane Review on thoracic imaging tests for the diagnosis of COVID-19 has been updated, for a third time, in May 2022. It brings together evidence on the accuracy of chest (thoracic) imaging (computed tomography (CT), X‐ray and ultrasound) in diagnosing COVID‐19 in people with suspected infection. The review also considers the accuracy of chest imaging for screening asymptomatic people.
This is one of a suite of Cochrane ‘living systematic reviews’ summarising evidence on the accuracy of different imaging tests and diagnostic features in people regardless of their symptoms, grouped according to the research questions and settings.
Screening people with suspected COVID-19
The review includes 94 studies with 37,631 people with suspected COVID-19, of whom just over half had a final diagnosis of COVID-19. Most of the studies looked at chest CT.
Chest CT: in people with suspected COVID
- Chest CT correctly diagnosed COVID‐19 in 87% of people who had COVID‐19.
- However, it incorrectly identified COVID‐19 in 21% of people who did not have COVID‐19.
Chest X‐ray: in people with suspected COVID
- Chest X‐ray correctly diagnosed COVID‐19 in 73% of people who had COVID‐19.
- However, it incorrectly identified COVID‐19 in 27% of people who did not have COVID‐19.
Lung ultrasound: in people with suspected COVID
- Lung ultrasound correctly diagnosed COVID‐19 in 87% of people with COVID‐19.
- However, it incorrectly diagnosed COVID‐19 in 24% of people who did not have COVID‐19.
Screening asymptomatic people
The review also included 10 studies with 3548 people who had no symptoms (asymptomatic), of whom 364 (10%) had a final diagnosis of COVID‐19.
The results suggest CT correctly diagnosed COVID‐19 in only 56% of people who actually had COVID‐19 and incorrectly identified COVID‐19 in 8% of people who did not have COVID‐19.
Overall, the evidence so far suggests that chest CT and ultrasound are better at ruling out COVID-19 infection than distinguishing it from other respiratory problems. So, their usefulness may be limited to excluding COVID‐19 infection rather than distinguishing it from other causes of lung infection. In addition, chest imaging may only correctly diagnose COVID-19 in just over half of asymptomatic people who do actually have COVID. The authors’ confidence in the evidence is limited because the studies differed from each other, used different methods to report their results and very few studies directly compared one type of imaging test with another.
Measures to control the spread of COVID-19
School-based measures to contain the COVID-19 pandemic
Shutting schools was one of the earliest responses to the pandemic in many countries. As well as potential benefits of this strategy for limiting the spread of infection there are many potential harms, including worsening health and wellbeing for children and widening inequalities. Alternatives to school closure are also being adopted, including the wearing of face masks, hand hygiene, changes to school activities, improved ventilation systems and screening. It is important to have evidence of the effectiveness of these different measures to inform policy and practice.
A Cochrane Review (published in January 2022) explored the potential benefits and harms of measures implemented in the school setting to contain the COVID‐19 pandemic.
The key messages are that:
“Reopening schools or keeping schools open while having a broad range of measures in place can reduce transmission of the virus that causes COVID‐19. Such measures can also reduce the number of people who will need to go to hospital due to developing COVID‐19. We still know very little about other consequences of these measures, such as those linked to education, resources, and physical or mental health, as this knowledge is mostly based on studies modelling the real world. More studies set in the real world using real‐world data are needed.”
See also:
- A scoping review (published December 2020) on measures implemented in the school setting to contain the COVID‐19 pandemic, mapping the existing evidence.
- A scoping review (published June 2022) Unintended consequences of measures implemented in the school setting to contain the COVID‐19 pandemic: a scoping review mapping the existing evidence about possible unintended outcomes of school-based measures to contain COVID-19. For example, educational consequences such as changes in school performance; psychosocial outcomes such as anxieties about going to school; physical outcomes such as hand eczema due to increased handwashing; environmental consequences such as reduced air quality in classrooms; and socio-economic consequences such as economic burden on families.
- Cochrane Clinical Answers:
Quarantine for controlling COVID-19
A Cochrane rapid review on quarantine alone or in combination with other public health measures to control COVID-19 was published in April 2020 and updated in September 2020. It is the focus of this blog: “Quarantine for controlling COVID-19 (coronavirus). New Cochrane evidence.”

Watch a video of the lead author summarising the review’s findings. You might also be interested in this blog ““Stay at home” rules: what makes people more likely to stick to quarantine?“, which looks at two non-Cochrane rapid reviews from researchers at King’s College London.
Contact tracing
Contact tracing aims to reduce transmission of infection by identifying people who have been in contact with someone who has it, so that they can isolate. A Cochrane rapid review published in August 2020 looked at evidence on digital contact tracing technologies in epidemics.
The review highlights an evidence gap, the authors concluding that “the effectiveness of digital solutions is largely unproven as there are very few published data in real‐world outbreak settings.”
Personal Protective Equipment (PPE) for healthcare workers
Two Cochrane reviews contribute to the evidence base on PPE for healthcare workers and we have a blog about these: “Personal protective equipment (PPE) for healthcare workers: new Cochrane evidence“. They look at Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff (May 2020) and Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis (April 2020).

For the review on PPE there is a Cochrane Clinical Answer. For the review on barriers and facilitators there is podcast and a Cochrane Clinical Answer. Evidence Synthesis Ireland and Cochrane Ireland have also created an infographic summarising key messages.
Preventing or reducing COVID-19 infections in long term care facilities (care homes)
A Cochrane Review ‘Can non‐medicinal measures prevent or reduce SARS‐CoV‐2 infections in long term care facilities?’ (published September 2021) included 22 studies exploring this question. 11 of the studies were observational, i.e. they used real-world data, and 11 were modelling studies, i.e. they used mathematical prediction.
There were four main types of measures.
1) Entry regulation measures to prevent residents, staff or visitors introducing the virus into the facility
Examples include: staff confining themselves with residents, quarantine for newly‐admitted residents, testing new admissions, not allowing the admission of new residents, and preventing visitors from entering facilities.
Most studies showed such measures may be beneficial, but some studies found that there may be no little or no effect or unwanted effects, such as delirium and depression among residents when visitors were restricted.
For more information: What are the effects of COVID‐19 entry regulation measures in long‐term care facilities (LTCFs)?
2) Contact‐regulating and transmission‐reducing measures to prevent people passing on the virus
Examples include: wearing masks or personal protective equipment (PPE), social distancing, extra cleaning, reducing contact between residents and among staff, and placing residents and staff in care groups and limiting contact between groups.
Some measures may be beneficial, but the evidence is often very uncertain.
For more information: What are the effects of contact regulation and transmission‐reducing measures on COVID‐19 in long‐term care facilities (LTCFs)?
3) Surveillance measures designed to identify an outbreak early
Examples include: regular testing of residents or staff regardless of symptoms, and symptom‐based testing
Routine testing of residents and staff may reduce the number of infections, hospitalisations, and deaths among residents (although the evidence on the number of deaths among staff was less clear).
Testing more often, getting test results faster, and using more accurate tests may have more beneficial effects.
For more information: What are the effects of COVID‐19 surveillance measures in long‐term care facilities (LTCFs)?
4) Outbreak control measures to reduce the consequences of an outbreak
Examples include: isolation of infected residents, and separating infected and non‐infected residents or staff caring for them.
These measures may reduce the number of infections and the risk of outbreaks in facilities, but often the evidence is very uncertain.
For more information: What are the effects of COVID‐19 outbreak control measures in long‐term care facilities (LTCFs)?
A combination of different measures may be effective in reducing the number of infections and deaths.
Antimicrobial mouthrinses and nasal sprays to protect healthcare workers and patients at risk of COVID-19
Antimicrobial mouthrinses and nasal sprays have the potential to help people with COVID-19 fight infection and prevent them infecting healthcare workers who care for them. They might also offer some protection to healthcare workers, especially if they use them before doing aerosol-generating procedures, such as drilling teeth.
Three new Cochrane Reviews were published in September 2020 looking at different aspects of this. There is a helpful summary of all three in this Cochrane Oral Health Editorial base blog Antimicrobial mouthrinses and nasal sprays to protect healthcare workers and patients at risk of COVID-19, which also has links to each of the reviews. No completed studies were found for any of the reviews, so this is currently an evidence gap, but there are ongoing studies for two of these three reviews and all will be updated.
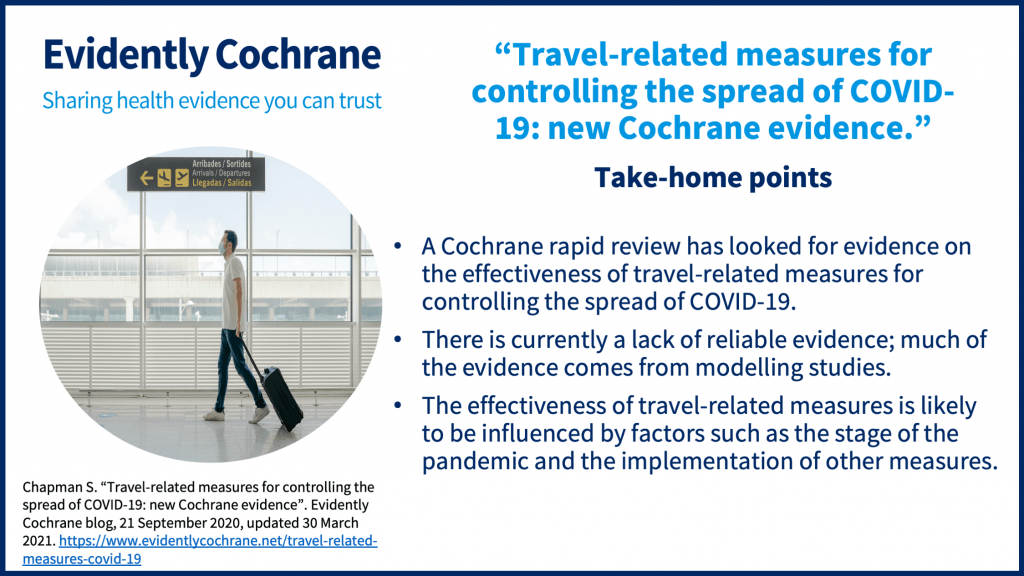
Travel-related measures
A Cochrane rapid review, published in September 2020, looked at travel-related control measures to contain the COVID-19 pandemic. Not surprisingly, it highlighted uncertainty about their effectiveness and a lack of reliable and ‘real-life’ evidence. The review was updated in March 2021.
Jacob Burns, lead author of this update explains, “In this update we identified a much expanded evidence base related to international travel control measures to contain the COVID-19 pandemic, with 38 additional studies focusing on COVID-19 identified. Many of the studies were similar with regard to scope and methods, and overall the conclusions of the updated review remain largely the same. Some aspects of the evidence base, however, were improved – for example, we identified studies from further parts of the world that were not represented in the original review, including African and Eastern Mediterranean regions. Additionally, we identified more studies evaluating entry and/or exit screening measures at real-world ports of entry.”
You can read about the review in this blog “Travel-related measures for controlling the spread of COVID-19: New Cochrane evidence“.
2
Things that increase people’s risk of suffering from severe COVID-19 illness
Obesity
A Cochrane Review Obesity as an independent risk factor for COVID‐19 severity and mortality (published May 2023) looked at whether obesity increases the risk of suffering from severe COVID-19 illness. The authors found that:
- extreme obesity (BMI, or Body Mass Index greater than 40 kg/m2) – compared with having a ‘normal’ BMI (18.5 to 24.9 kg/m2) – increases the chance that a person with COVID will need a breathing tube, and may increase a person’s chances of:
- being hospitalised;
- being admitted to intensive care due to COVID‐19;
- dying;
- obesity with a BMI between 30-40 kg/m2 probably increases the chance of a person requiring a breathing tube, but may have little to no impact on their risk of dying.
- “evidence suggests that the higher a person’s BMI gets, the higher the chance that they will suffer from severe COVID‐19 disease”.
This is based on 171 eligible studies; with 149 of those studies (including 12,045,976 people) providing data for at least one of the analyses.
2
Preventing COVID-19
COVID-19 vaccines
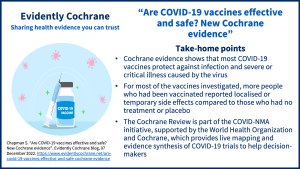
A Cochrane Review Efficacy and safety of COVID-19 vaccines (published December 2022) concludes that “Compared to placebo, most vaccines reduce, or likely reduce, the proportion of participants with confirmed symptomatic COVID‐19, and for some, there is high‐certainty evidence that they reduce severe or critical disease. There is probably little or no difference between most vaccines and placebo for serious adverse events.”
The key points are covered in our blog Are COVID-19 vaccines effective and safe? New Cochrane evidence.
Are laboratory‐made COVID‐19‐specific monoclonal antibodies effective to prevent COVID‐19 in adults?
A Cochrane Review SARS‐CoV‐2‐neutralising monoclonal antibodies to prevent COVID‐19 (published June 2022) has explored whether COVID‐19‐specific monoclonal antibodies are effective and safe at preventing COVID‐19 in people who have either been exposed to the virus, or who are at high risk of being exposed.
Antibodies are made by the body as a defence against disease. They can also be produced in a laboratory, from cells taken from people who have recovered from a disease.
Antibodies that are designed to target only one specific protein – in this case, a protein on the virus that causes COVID‐19 – are ‘monoclonal’. They attach to the COVID‐19 virus and stop it from entering and reproducing in human cells. This may help to fight the infection.
The authors found four studies with 9749 people, comparing monoclonal antibodies (e.g. bamlanivimab, tixagevimab/cilgavimab, and casirivimab/imdevimab) with placebo (dummy treatment), another treatment, or no treatment to prevent COVID‐19 in people of any age.
Key messages:
In people who are at high risk of being exposed to COVID-19, but haven’t yet been exposed to the virus:
- tixagevimab/cilgavimab probably reduces number of people infected with COVID‐19 and development of COVID‐19 symptoms, and may reduce the number of people admitted to hospital;
- casirivimab/imdevimab may reduce the number of people infected with COVID‐19 and development of COVID‐19 symptoms, but may increase number of unwanted effects (any severity) slightly.
In people who have been in contact with an infected person:
- bamlanivimab probably reduces the number of people infected with COVID‐19;
- casirivimab/imdevimab reduces the number of people infected with COVID‐19 and development of COVID‐19 symptoms, and reduces number of unwanted effects (any severity).
It is important to note that all the studies were carried out before the vaccine roll-out (so all participants were unvaccinated at the start of the studies) and before the emergence of the Omicron variant. As a result, it’s uncertain how relevant these findings are today in the UK. The review authors identified four ongoing studies which may give greater clarity. They will update the review in time.
.
3
The impact of the pandemic on other areas of health and wellbeing
Resilience and mental health of frontline healthcare professionals
Working on the ‘front line’ as a health or social care professional during a pandemic is stressful and can negatively impact workers’ mental health. A Cochrane Review on interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic aimed to assess the effects of such interventions and explore things that make it easier or harder to implement them, through both qualitative and qualitative evidence.
Intervention effects remain uncertain. They were explored in just one study. All 16 studies in the review had some limited evidence on things that might help interventions to be successfully delivered. This review highlights a need for robust evaluation of interventions and the review authors suggest that the current pandemic provides unique opportunities for doing so.
Interventions for heavy menstrual bleeding
Pandemics disrupt healthcare provision. With this in mind, a Cochrane overview of reviews (July 2020) has been done on interventions commonly available during pandemics for heavy menstrual bleeding. You can see summaries of the review here, including an infographic to help women make choices about treatment. There is also a podcast about this review and two Cochrane Clinical Answers.
Routine vaccinations during the pandemic
The World Health Organization (WHO) has emphasized the importance of keeping up with routine vaccinations during the pandemic, advice endorsed by Public Health England. A Cochrane Review on vaccines for measles, mumps, rubella and varicella in children (published April 2020) was discussed in this blog: “MMR vaccines: do they work and are they safe?“.
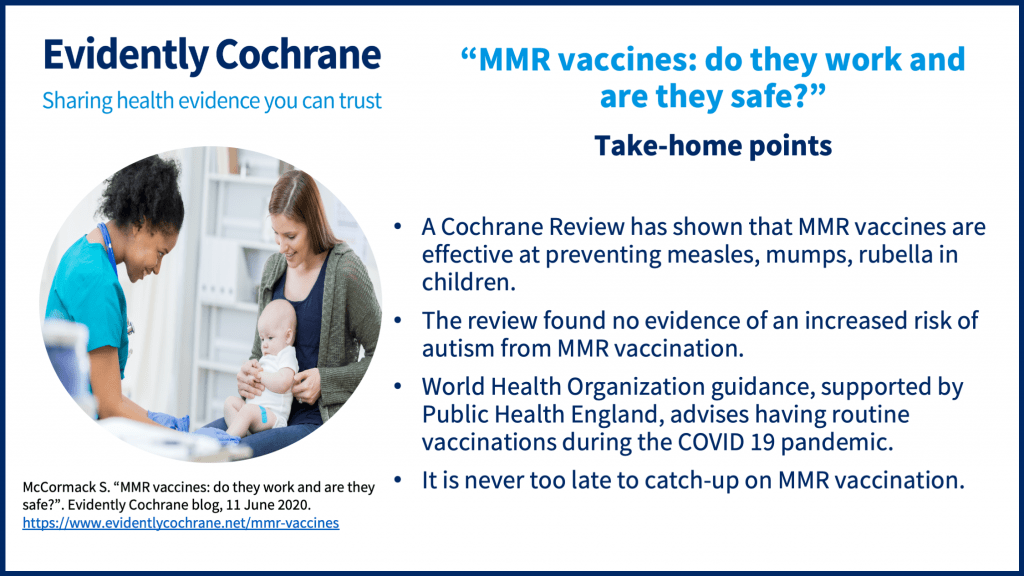
There are two Cochrane Clinical Answers related to this review.
Social isolation and loneliness in older people
With restrictions imposed during the pandemic increasing isolation for many, a Cochrane rapid review (May 2020) looked for evidence on video calls for reducing social isolation and loneliness in older people. We discuss the review in this blog: “Loneliness in older people: could video calls help?”

There is also a podcast and a Cochrane Clinical Answer.
Quitting smoking to improve respiratory health
Given the current threat from COVID-19, an acute respiratory infection, there has never been a better time to stop smoking, and the World Health Organization is urging people to do so. We have looked at evidence from a new Cochrane Special Collection, COVID-19: Effective options for quitting smoking during the pandemic, in this blog: “Smoking and coronavirus (COVID-19): time to quit.”

.
Preventing and treating persistent symptoms after COVID-19 infection
Plasmapheresis to remove amyloid fibrin(ogen) particles for treating post-COVID 19 syndrome (long COVID)
The cause of long COVID is debated. One theory is that it is caused by small clots in the blood, which authors of a set of laboratory studies that investigate them call ‘microclots’. However, the authors of the Cochrane Review Plasmapheresis to remove amyloid fibrin(ogen) particles for treating the post‐COVID‐19 condition (July 2023) note that they are not clots, and refer to them as amyloid and fibrin(ogen) particles, as they appear to contain these proteins. Amyloid fibrin(ogen) particles are found in healthy people and those with other diseases.
They aimed to assess the evidence of the safety and efficacy of plasmapheresis to remove amyloid fibrin(ogen) particles, in people with long COVID, from randomized controlled trials. They found none, and conclude that “In the absence of reliable research showing that amyloid fibrin(ogen) particles contribute to the pathophysiology of PCC, there is no rationale for plasmapheresis to remove amyloid fibrin(ogen) particles in PCC. Plasmapheresis for this indication should not be used outside the context of a well‐conducted randomized controlled trial.”
Persistent problems with sense of smell after COVID-19 infection
A Cochrane Review on Interventions for the prevention of persistent smell disorders (olfactory dysfunction) after COVID‐19 infection and another on Interventions for the treatment of persistent smell disorders after COVID‐19 infection were updated in September 2022. There remains little very little evidence on the benefits or harms of interventions to prevent or treat problems with sense of smell after COVID-19. These are ‘living systematic reviews’ to which the authors can add new research as it becomes available.
See also the Cochrane Clinical Answers:
.
8
Vaccination
Increasing uptake of COVID-19 vaccines
Scoping reviews aim to map the existing evidence on a topic. The authors of Interventions to increase COVID‐19 vaccine uptake: a scoping review (August 2022) aimed to find out which interventions to increase COVID‐19 vaccine uptake have been – or are currently – evaluated.
They found:
- 96 studies (35 of which are ongoing; 61 studies had published results)
- The interventions tested in these studies are very diverse. Many studies used communication strategies to convince people to get vaccinated against Covid. Interventions that included information on vaccination or a mixture of different strategies were also often used.
- The participants in the studies were diverse. For example, studies addressed healthcare workers, ethnic minorities in the USA, students, soldiers, villagers, at‐risk patients, or the general population.
- However, there is a lack of research focusing on:
- lower‐middle‐income countries
- children
- how different interventions compare
You can see an overview of the studies in this interactive scoping map.
This review cannot answer the question ‘which interventions are most effective to increase willingness to have the COVID‐19 vaccine?’, but it can be the foundation of subsequent systematic reviews addressing this important question – as well as highlighting important gaps for researchers to address.
There is a related Cochrane Clinical Answer: What evidence is available on interventions to increase COVID-19 vaccine uptake or decrease COVID-19 vaccine hesitancy?
The effects of COVID-19 vaccines in vulnerable people
The authors of Immunity after COVID‐19 vaccination in people with higher risk of compromised immune status: a scoping review (August 2022) aimed to find out which studies on the most commonly used COVID‐19 vaccines in vulnerable groups have been published, and which outcomes they’ve reported.
They found:
- 318 studies, mostly from high‐income countries and including adults.
- Most commonly, studies have looked at people with haematological malignancies (cancers that affect the blood, bone marrow, and lymph nodes – such as lymphoma or leukaemia) and solid tumours, followed by people receiving dialysis and kidney transplants, rheumatic diseases, and others. 31 studies included pregnant or breastfeeding women.
- The most reported outcomes are related to immunogenicity (how well a vaccine works, or the ability to stimulate the development of antibodies).
You can see an evidence gap map, compiled from their scoping review findings intended to steer future research. You can also see a related Cochrane Clinical Answer: What evidence is available on vaccines against SARS-CoV-2 in people at higher risk of lower immune response?
In itself, this review can’t tell us about the safety and effectiveness of vaccines in vulnerable populations. However, based on their findings, the authors decided to conduct two detailed systematic reviews on haematological malignancies and kidney transplant recipients.
One of these – exploring the safety and effectiveness of COVID vaccines for people with haematological malignancies – has now been published (as a non-Cochrane systematic review). It brings together data from 57 studies with 7393 patients.
They found that adverse events were “rarely reported”. Regarding effectiveness, the authors say that patients with haematological malignancies “present impaired… immune response to COVID-19 vaccination”. It’s likely that this is “due to disease and treatment-associated immune impairment”. As a result, “new approaches to avert severe infection are urgently needed for this vulnerable patient population that responds poorly to current COVID-19 vaccine regimens”.
Keeping up with the rapidly changing nature of the pandemic is challenging. For example, none of the studies have looked at how effective vaccines are against the omicron variant.
The other non-Cochrane review, looking at the effects of COVID vaccine boosters in kidney transplant recipients, has not yet been published.
.
Coronavirus (COVID-19): Special Collections
The Special Collection on quitting smoking during the pandemic is one of eight Cochrane Special Collections on COVID-19. Developed with experts from our global Cochrane network, they are based on World Health Organization interim guidance, and continuously updated.
.
Coming up…
Like the reviews themselves, we update our blogs to reflect the latest evidence. There are new reviews coming up, as well as updates of existing reviews, so check back for additions to this blog.
We also welcomed news (March 2021) of two new clinical trials which have been launched in the UK to investigate potential preventative treatments for the most clinically vulnerable (those with long-term underlying conditions and those in care homes) to prevent them catching COVID-19.
Keeping up to date
As well as coming back to this blog, you can find Cochrane resources and news on COVID-19 and this will also be continually updated.
Join in the conversation on Twitter with @CochraneUK @SarahChapman30 or leave a comment on the blog. Please note, we will not publish comments that link to commercial sites or appear to endorse commercial products. We welcome diverse views and encourage discussion but ask that comments are respectful and reserve the right to not publish comments we consider offensive.
Sarah Chapman and Selena Ryan-Vig have nothing to disclose.




I can’t find any Cochrane reviews on the efficacy of various covid vaccines. Are there no reviews? Surprises me there isn’t
Thank you for this question, which has led us to make enquiries. We understand that a review of the COVID vaccines is being prepared and should be available soon. Meanwhile, you might wish to see the joint Cochrane and WHO COVID-19 living network meta-analysis initiative which provides living maps of evidence on preventing and treating COVID-19 and maintains living syntheses of evidence, including on vaccines, which are updated every 2 weeks.
The living map of evidence on vaccines is here: https://covid-nma.com/vaccines/mapping/
The living synthesis of RCT evidence on vaccine effectiveness is here: https://covid-nma.com/vaccines/
The living synthesis of RCT evidence on vaccine effectiveness on variants of concern is here: https://covid-nma.com/vaccines/variants/
The living synthesis of observational studies evidence on vaccine effectiveness on variants of concern is here: https://covid-nma.com/vaccines/os_vaccines/
The Cochrane protocol for this initiative is here:
Boutron I, Chaimani A, Devane D, Meerpohl JJ, Rada G, Hróbjartsson A, Tovey D, Grasselli G, Ravaud P. Interventions for the prevention and treatment of COVID‐19: a living mapping of research and living network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No.: CD013769. DOI: 10.1002/14651858.CD013769
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013769/full
We hope this helps.
Best wishes,
Sarah Chapman [Editor]
There is one coming soon and we will be sure to add it here.
I found this blog incredibly useful to present what we have done in Cochrane and why. Thank you very much Sarah!
Thats great to hear Karla, thank you!
I liked very much those informations!
I’m very glad you found it useful.